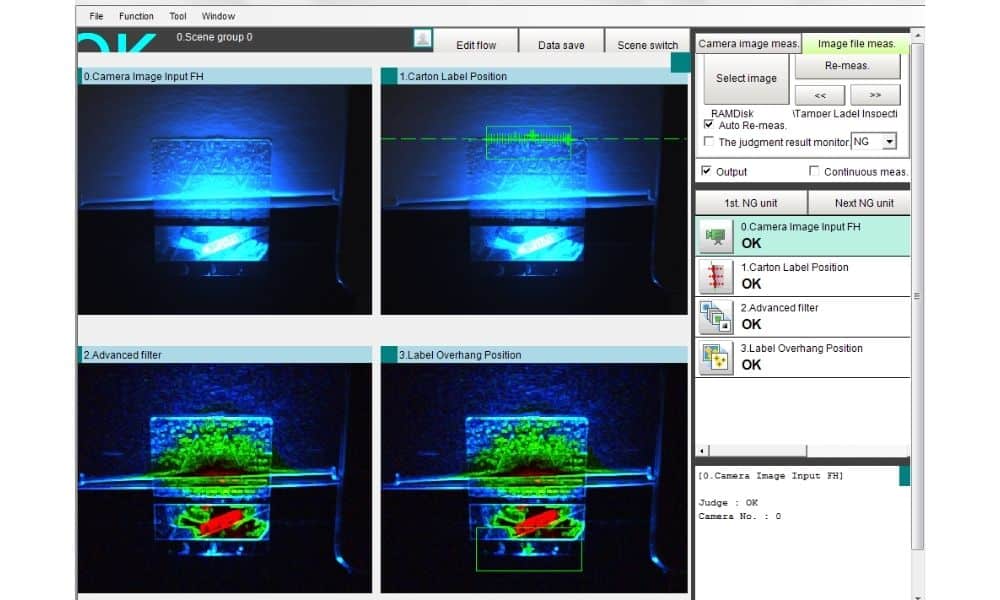
The Background: In order to comply with regulations requiring manufacturers of prescription medicines to include an anti-tampering device on packaged products, a leading Irish pharmaceutical manufacturer took the decision to apply transparent tamper evident labels on their products. As part of final product inspection the manufacturer decided that they required an automatic inspection system to check for presence of the tamper evident labels on each box.
The Solution: Detecting the presence of transparent labels on a white box is quite challenging using standard sensor technology so it was decided that a vision system was the most reliable solution for this application. Based on an Omron platform the proposed solution included an FH controller along with two standard resolution monochrome cameras and external lighting. The integrated services division of ATC Automation installed and commissioned the vision system ensuring the presence of the tamper evident label on the top and side of the packaging. The system was configured so that the line was stopped if the tamper evident label was either missing or incorrectly applied.
The Future: The installation of the vision system now ensures that the pharmaceutical manufacturer can reliably detect the presence of the tamper evident labels and comply with forthcoming medicine counterfeit regulations. The system can inspect 200 boxes per minute which meets with both current and forecasted production requirements.